Abstract
Background: Heparin-induced thrombocytopenia (HIT) is a life-threatening complication in which antibodies to platelet factor 4 complexed to heparin lead to platelet overactivation resulting in a hypercoagulable state. Treatment begins with stopping all sources of heparin and initiating rapid-acting non-heparin anticoagulation. Bivalirudin is a direct thrombin inhibitor (DTI) used off-label for the prevention and treatment of HIT. It is primarily enzymatically cleared; however, dose reductions are required in renal impairment. Despite its widespread off-label use in HIT, there is a paucity of data for dosing bivalirudin in patients with renal dysfunction and obesity. Here we report the results of a medication use evaluation (MUE) investigating the safety and efficacy of dosing bivalirudin based on renal function and body mass index (BMI).
Methods: We conducted a retrospective MUE including patients 18-89 years of age receiving bivalirudin with ≥3 DTI levels between June 2019 and December 2021. Patients receiving bivalirudin for percutaneous coronary intervention were excluded. Creatinine clearance was measured with the Cockcroft-Gault equation. The primary outcome was time to therapeutic anticoagulation (TTA) defined as the time in hours (hr) between bivalirudin initiation and the first of two consecutive therapeutic DTI levels. Secondary outcomes included average initial therapeutic dose, time in therapeutic range (TTR) calculated as the percentage of therapeutic DTI levels, incidence of clinically significant bleeding, and average number of dose changes before reaching therapeutic goal.
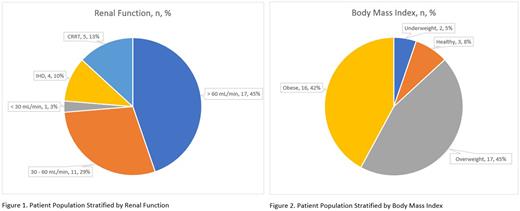
Results: Thirty-eight patients (21 females, 55%) with a mean (SD) age of 60.3 (13.5) years were included, 12 of which had a history of or confirmed HIT by serotonin release assay. The remaining patients had suspected HIT or coagulation abnormalities precluding the use of heparin. One patient was classified as an outlier and excluded due to requiring an abnormal number of dose adjustments. Among this population, 17 (45%) had normal renal function (CrCl >60 mL/min), 11 (29%) had mild renal impairment (CrCl 30-60 mL/min), 1 (3%) had moderate renal impairment (CrCl <30 mL/min), 4 (10%) were on intermittent hemodialysis (IHD), and 5 (13%) were on continuous renal replacement therapy (CRRT). Stratified by BMI, 2 (5%) were underweight (BMI <18.5 kg/m2), 3 (8%) were healthy (BMI 18.5-24.9 kg/m2), 17 (45%) were overweight (BMI 25.0-29.9 kg/m2), and 16 (42%) were obese (BMI ≥30 kg/m2).
There was no statistically significant difference in TTA based on renal function or BMI. However, clinically, patients on IHD or CRRT trended toward having a shorter TTA compared to patients with normal, mildly impaired, and moderately impaired renal function (28.2 ± 9.0 or 28.9 ± 16.3 hr vs. 40.0 ± 27.6, 36.7 ± 31.6, and 42.9 hr, respectively). Obese and overweight patients trended toward a shorter TTA compared to healthy and underweight patients (31.4 ± 28.9 and 32.8 ± 17.4 hr vs. 62.3 ± 35.6 and 54.8 ± 5.0 hr, respectively).
The difference in TTA may be affected by lab turnaround time (TAT). To adjust for lab TAT, the average number of dose adjustments was assessed. There was no difference in the number of dose adjustments based on renal function. In contrast, overweight and obese patients trended toward requiring fewer dose changes compared to underweight and healthy patients. This trend aligned with overweight and obese patients achieving a statistically significantly higher TTR compared to underweight and healthy patients (55.5 vs. 34.4%, p = 0.02).
Based on bivalirudin's standard renal dose adjustments, patients with a CrCl >60 mL/min had a statistically significantly larger average initial therapeutic dose compared to those with a CrCl ≤60 mL/min (0.114 vs. 0.069 mg/kg/hr, p < 0.001). However, this difference was not observed based on BMI. Incidences of clinically significant bleeding were not observed to have affected TTA, TTR, average initial therapeutic dose, or number of dose changes before reaching therapeutic anticoagulation.
Conclusion: While our data are consistent with bivalirudin requiring renal dose adjustments, it was observed that underweight and healthy BMI patients required more dose adjustments and took longer to achieve therapeutic anticoagulation. More studies are needed to determine if these patients would benefit from a higher initial dosing strategy. This study was limited due to the sample size.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.